Covid 19 Antibody Test Kit Availability - Covid-19 Realtime Info
Its not widely available yet.

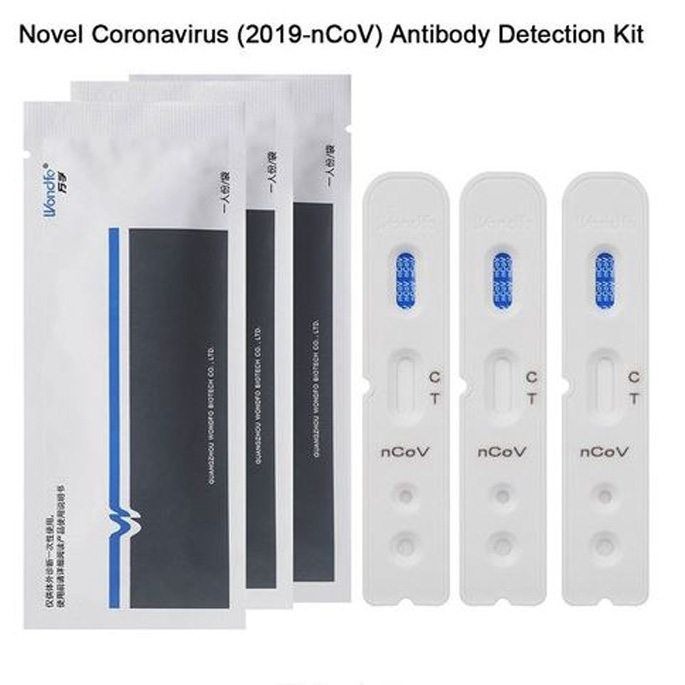
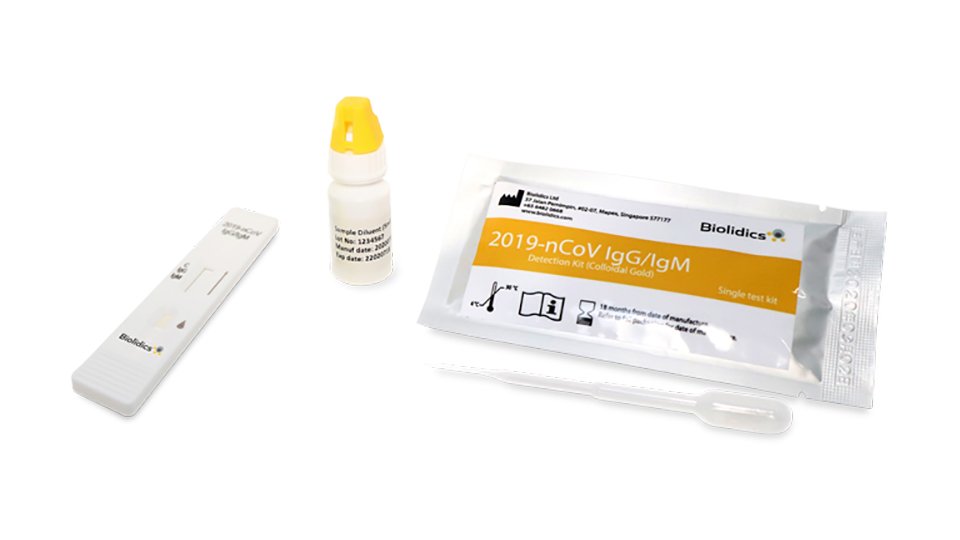
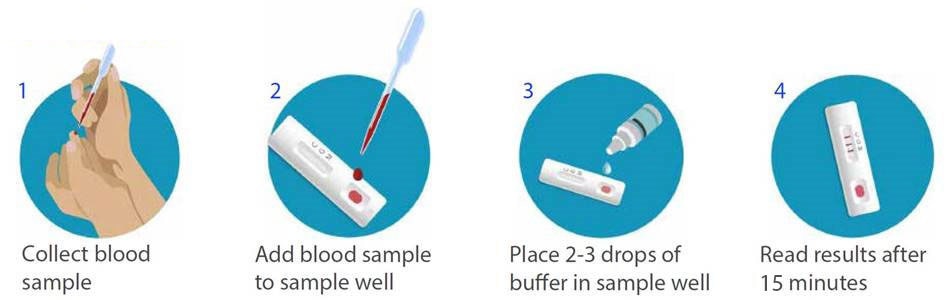
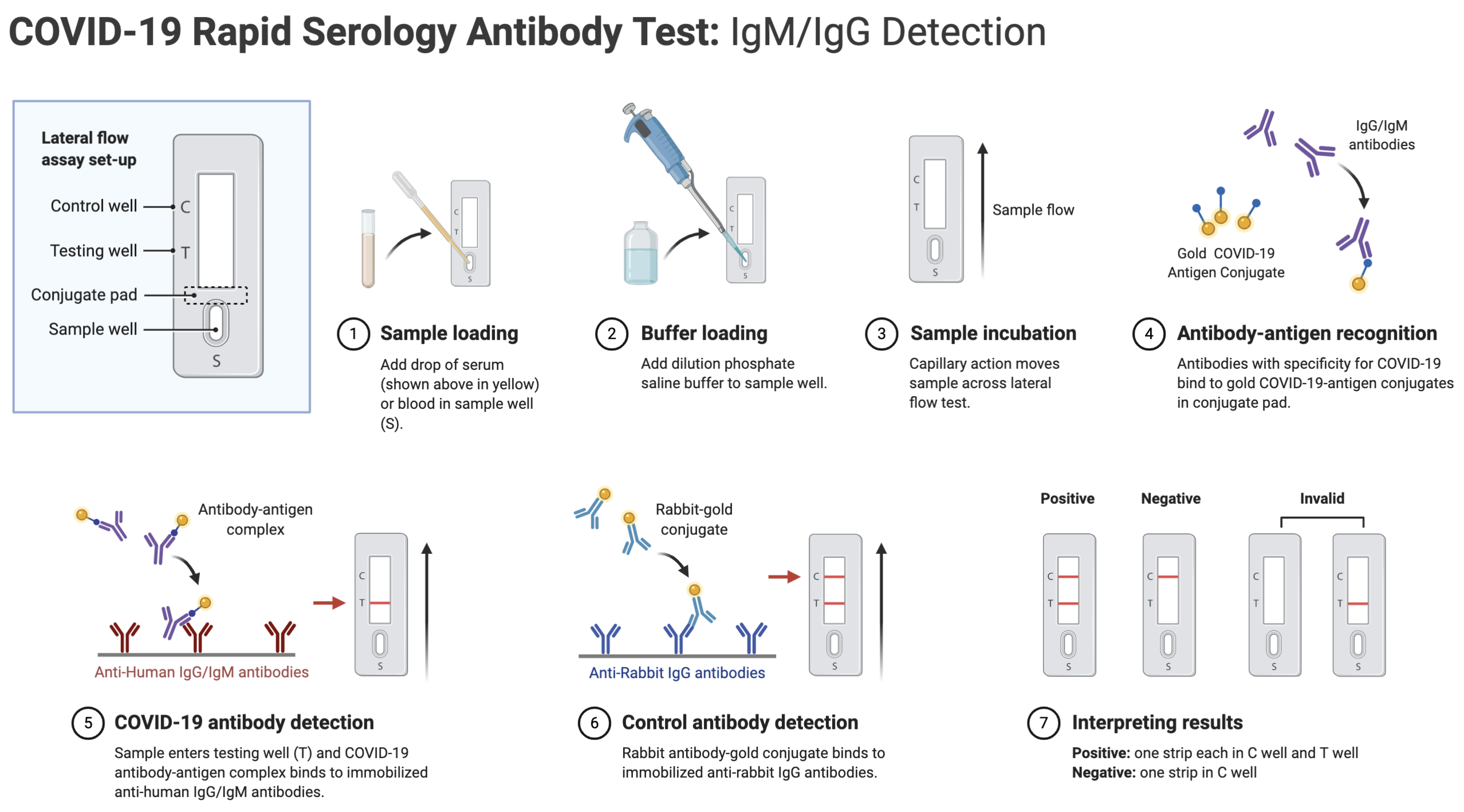
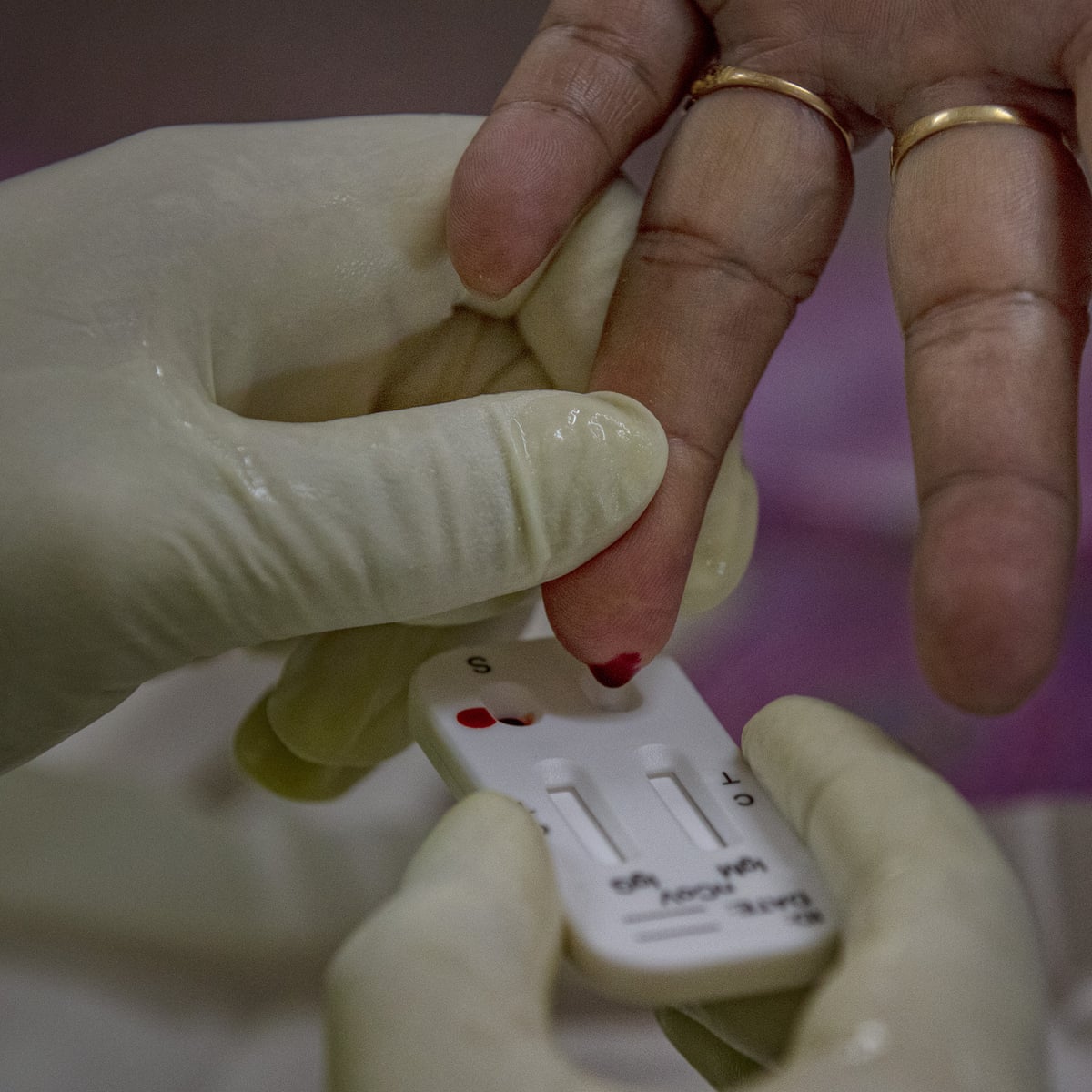
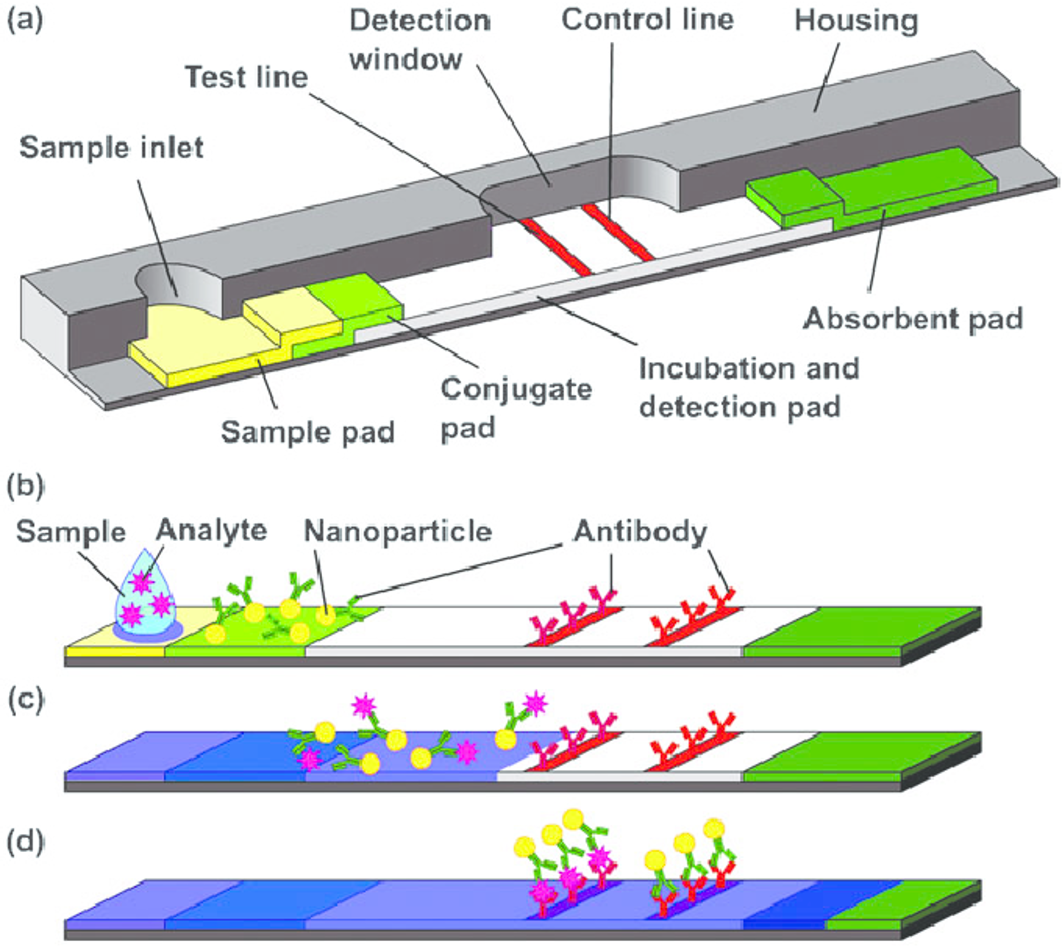
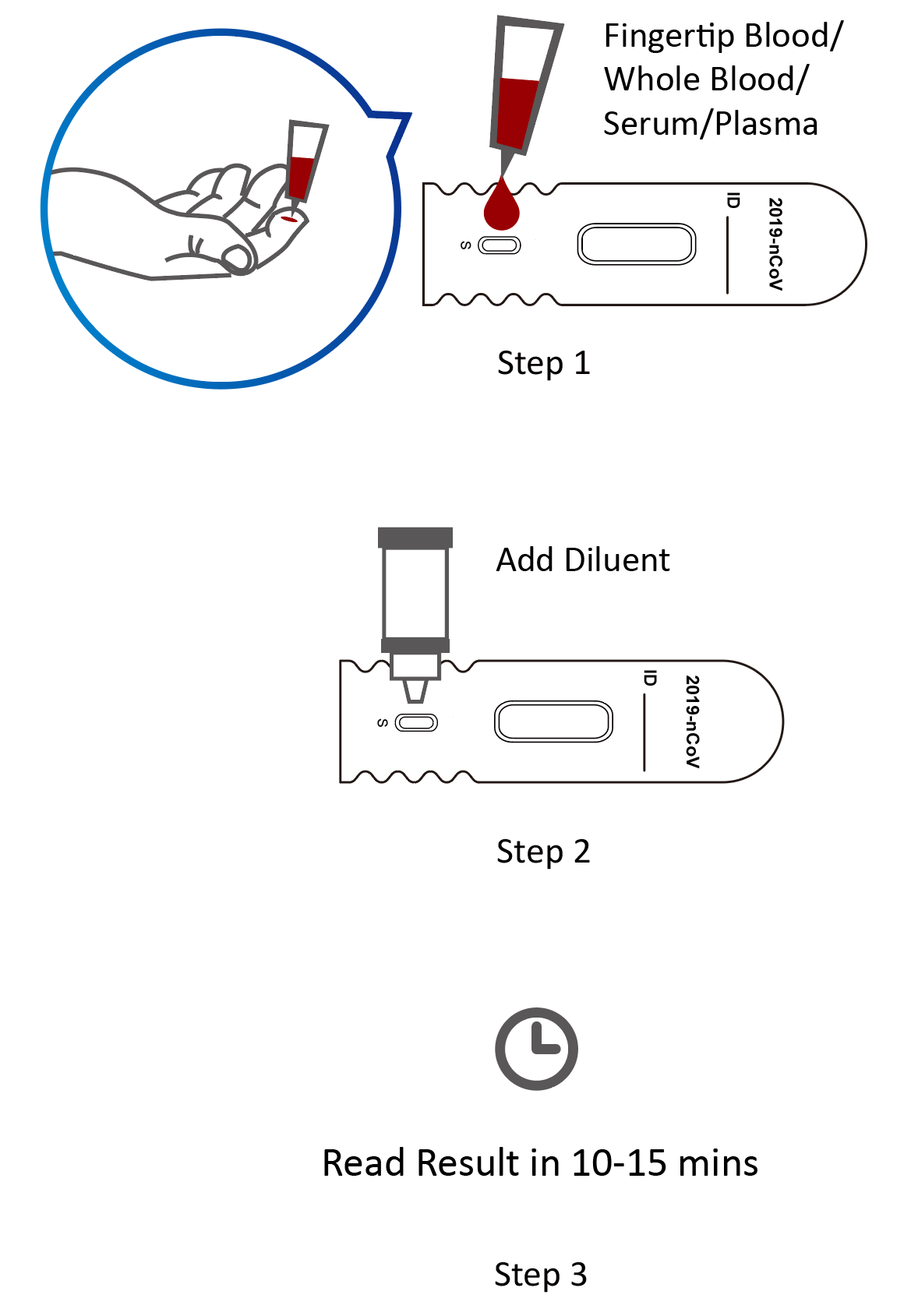
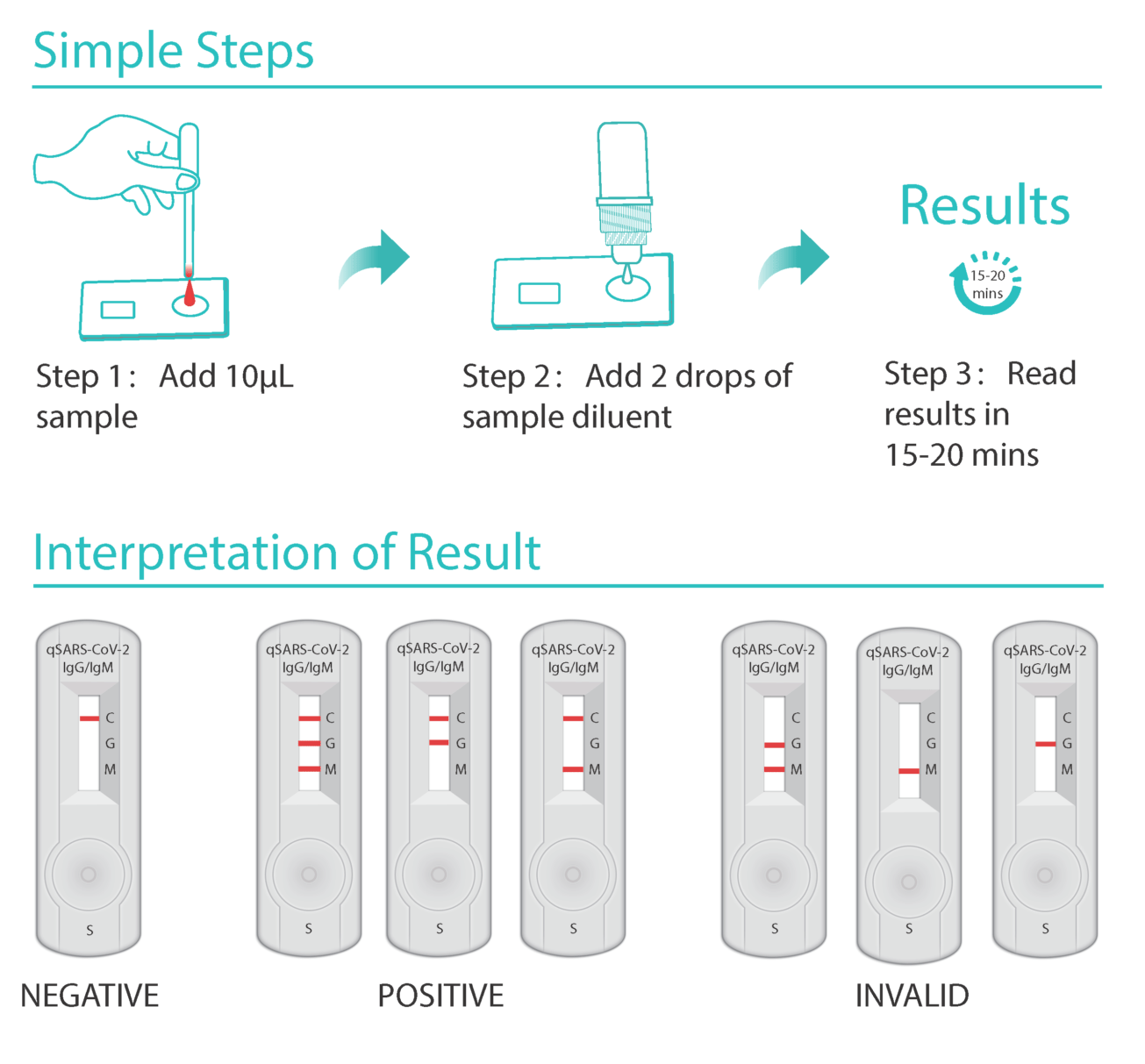
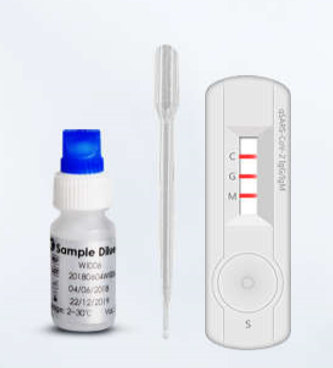
Covid 19 antibody test kit availability. These specially designed test kits are rapid chromatographic immunoassays configured like a home pregnancy test that are designed to detect igm and igg antibodies for covid 19 and sars cov 2 detection in the blood. This antibody test has over 95 specificity and sensitivity to covid 19. The blood tests for covid 19 look for antibodies specific to this coronavirus. Get tested for novel coronavirus covid 19.
Please refer to the provider for details. In response to the coronavirus covid 19 pandemic aurora is now offering the igmigg antibody rapid test kit to equip healthcare workers for rapid covid 19 antibody detection. After 14 days of infection igg will generally appear in the blood. If you want to know if your body has developed this particular immune response join our study.
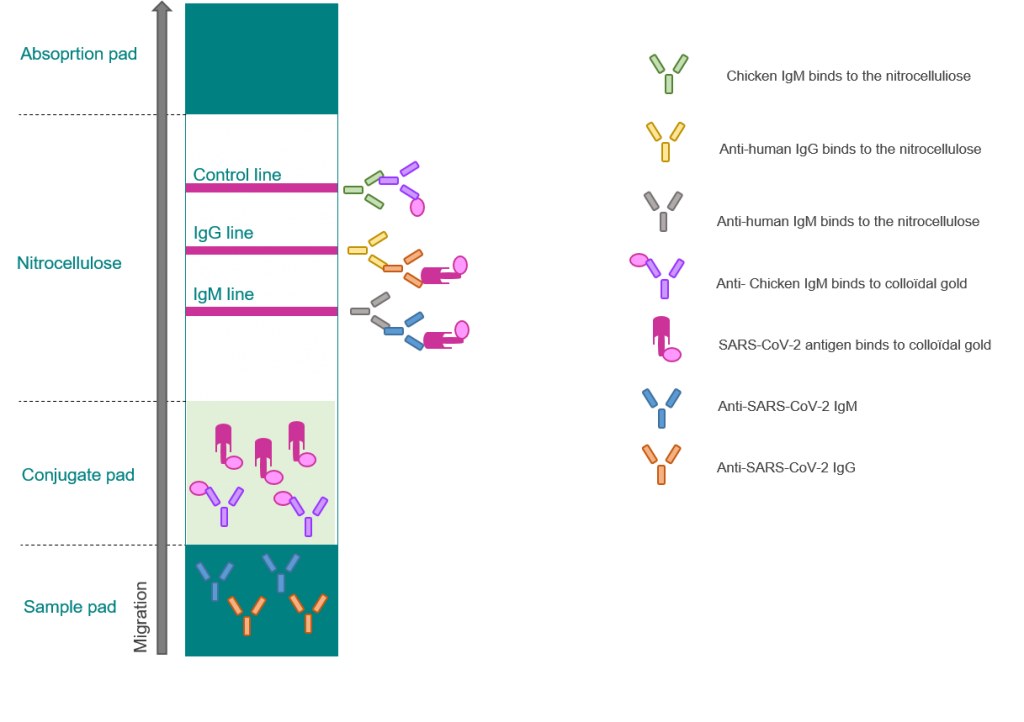
How does a covid 19 antibody test kit work. Covid 19 testing involves analyzing samples to assess the current or past presence of sars cov 2the two main branches detect either the presence of the virus or of antibodies produced in response to infection. Within 3 7 days of initial infection igm antibodies can generally be detected. Sars cov 2 igmigg antibody test kit different stages.
Choose from an continually updated list of testing providers online assessments and at home test kits. An antibody test can tell you if its likely youve had coronavirus before. An antibody test is a blood test to check if youve had coronavirus covid 19 before. 5 this testing has not been reviewed by the fda but its availability and performance has been determined to be appropriate with.
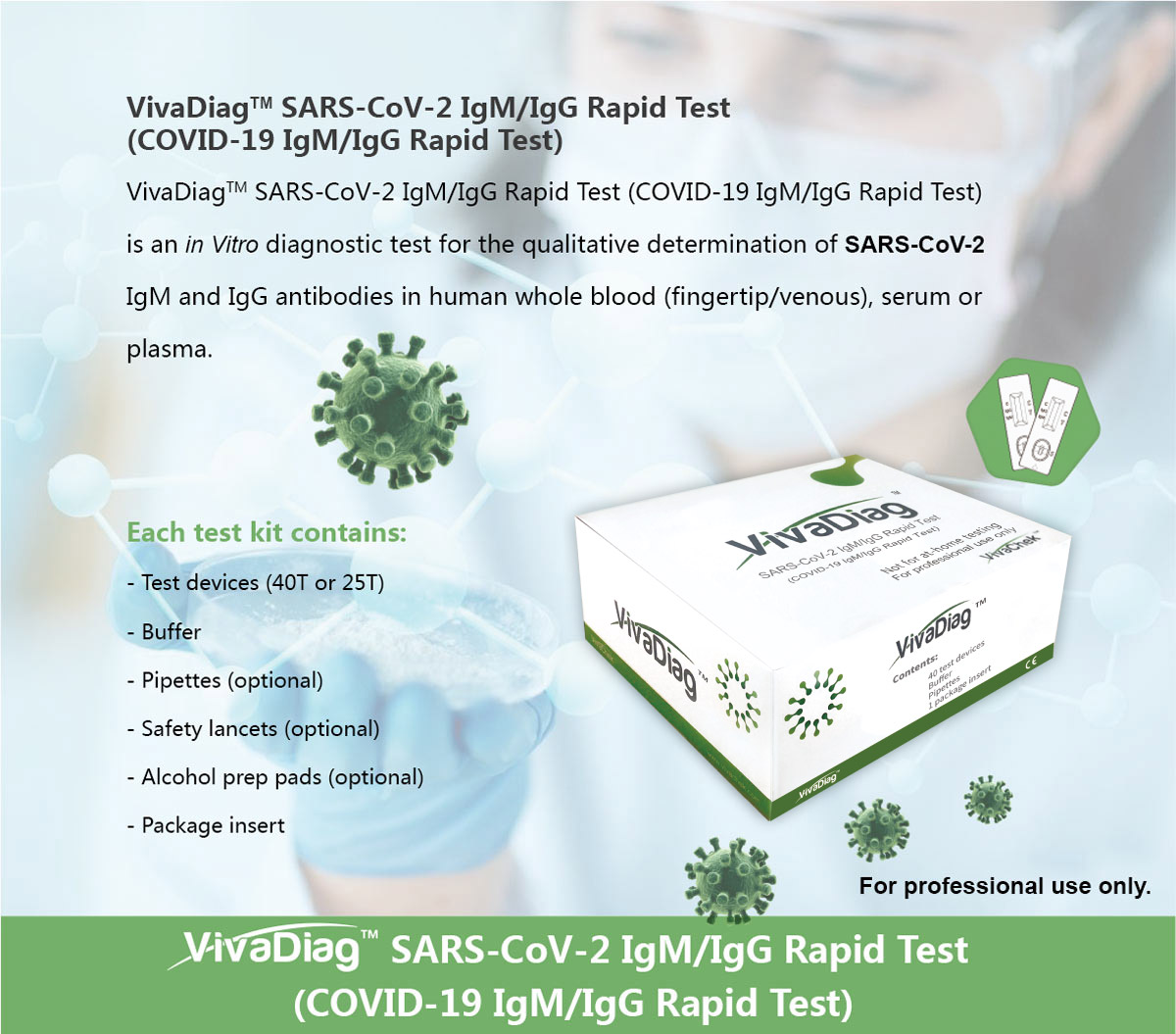
Sars cov 2 igmigg antibody test kit specificity. These kits are developed and manufactured by a us company that is a well respected provider of diagnostic test products for over 20 years. Coviant is a qualitative test for the detection of igg antibodies against sars cov 2 in human plasma and serumit is intended for use as an aid in identifying individuals with an adaptive immune response to sars cov 2 indicating recent or prior infection. Tests for viral presence are used to diagnose individual cases and to allow public health authorities to trace and contain outbreaks.
Granted emergency use authorization eua by the food and drug administration fda vessel medicals antibody test kits are available to be shipped nationwide for detection of covid 19. This covid 19 rapid test kit is suitable for the qualitative detection of sars cov 2 igmigg antibodies in human serum plasma or whole blood. Samples were collected from 197 serum samples of 40 patients with covid 19 on 1 7 days 8 14 days and at least 15 days after the onset of the disease to evaluate the coincidence rate of igm igg over time. The timing of coronavirus test results vary.
.jpg)